Controlling Crystal Growth Department Editor: Rita L. D’Aquino Chemical Engineering Magazine
Página 1 de 1.
 Controlling Crystal Growth Department Editor: Rita L. D’Aquino Chemical Engineering Magazine
Controlling Crystal Growth Department Editor: Rita L. D’Aquino Chemical Engineering Magazine
The formation of crystals requires the birth of new particles, also called nucleation, and the growth of these particles to the final product size. The driving force for both rates is the degree of supersaturation, or the numerical difference between the concentration of solute in the supersaturated solution in which nucleation and growth occurs vs. concentration of solute in a solution that is theoretically in equilibrium with the crystals.
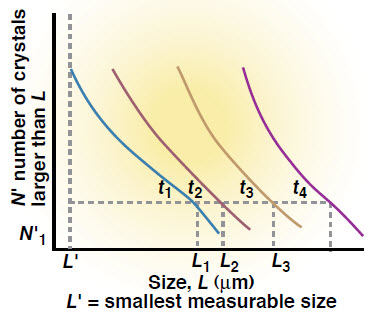
In a batch crystallizer, the crystal size distribution (CSD) is controlled by first seeding the initially supersaturated batch with a known number and size distribution of crystals, and then controlling the rate of evaporation or cooling (i.e., rate of energy transfer) so as to achieve a level of supersaturation that supports adequate crystal growth and an acceptable rate of nucleation.
The relationship between supersaturation and growth is linear, but that between nucleation and growth is raised to a power that is usually greater than one, making it difficult to grow large crystals when nucleation is occurring.
The following procedure describes how to achieve the optimal growth rate:
1. Screen the seeds at the beginning of the experiment to determine the cumulative number of crystals that are greater than a given size N’. Estimate NLi, the number of crystals of a given size (Lav) obtained from the screening:
In a batch crystallizer, the crystal size distribution (CSD) is controlled by first seeding the initially supersaturated batch with a known number and size distribution of crystals, and then controlling the rate of evaporation or cooling (i.e., rate of energy transfer) so as to achieve a level of supersaturation that supports adequate crystal growth and an acceptable rate of nucleation.
The relationship between supersaturation and growth is linear, but that between nucleation and growth is raised to a power that is usually greater than one, making it difficult to grow large crystals when nucleation is occurring.
The following procedure describes how to achieve the optimal growth rate:
1. Screen the seeds at the beginning of the experiment to determine the cumulative number of crystals that are greater than a given size N’. Estimate NLi, the number of crystals of a given size (Lav) obtained from the screening:
Read more:

 Temas similares
Temas similares» Solvent Selection Methodology Department Editor: Rita L. D’Aquino Chemical Engineering®
» Energy Efficiency in Steam Systems by Kate Torzewski, Department Editor - Chemical Engineering Magazine
» Preventing Runaway Reactions Department Editor: Rebekkah Marshall Chemical Engineering®
» Steam Tracer Lines and Traps by Scott Jenkins, Department Editor - Chemical Engineering
» Magazine ♦ Chemical Engineering ♦ May 2013
» Energy Efficiency in Steam Systems by Kate Torzewski, Department Editor - Chemical Engineering Magazine
» Preventing Runaway Reactions Department Editor: Rebekkah Marshall Chemical Engineering®
» Steam Tracer Lines and Traps by Scott Jenkins, Department Editor - Chemical Engineering
» Magazine ♦ Chemical Engineering ♦ May 2013
Página 1 de 1.
Permisos de este foro:
No puedes responder a temas en este foro.
 Índice
Índice
